what intermolecular forces must be overcome to melt solid i2?
11.2: Intermolecular Forces
- Page ID
- 21770
Learning Objectives
- To describe the intermolecular forces in liquids.
The properties of liquids are intermediate between those of gases and solids, just are more similar to solids. In contrast to intramolecular forces, such as the covalent bonds that concur atoms together in molecules and polyatomic ions, intermolecular forces hold molecules together in a liquid or solid. Intermolecular forces are mostly much weaker than covalent bonds. For example, it requires 927 kJ to overcome the intramolecular forces and break both O–H bonds in 1 mol of water, but it takes simply almost 41 kJ to overcome the intermolecular attractions and catechumen 1 mol of liquid water to h2o vapor at 100°C. (Despite this seemingly low value, the intermolecular forces in liquid h2o are among the strongest such forces known!) Given the big departure in the strengths of intra- and intermolecular forces, changes between the solid, liquid, and gaseous states well-nigh invariably occur for molecular substances without breaking covalent bonds.
The properties of liquids are intermediate between those of gases and solids, just are more similar to solids.
Intermolecular forces determine bulk properties, such every bit the melting points of solids and the boiling points of liquids. Liquids boil when the molecules have plenty thermal free energy to overcome the intermolecular attractive forces that concord them together, thereby forming bubbling of vapor within the liquid. Similarly, solids cook when the molecules acquire enough thermal energy to overcome the intermolecular forces that lock them into identify in the solid.
Intermolecular forces are electrostatic in nature; that is, they arise from the interaction betwixt positively and negatively charged species. Like covalent and ionic bonds, intermolecular interactions are the sum of both attractive and repulsive components. Considering electrostatic interactions fall off rapidly with increasing distance between molecules, intermolecular interactions are well-nigh important for solids and liquids, where the molecules are close together. These interactions go important for gases only at very high pressures, where they are responsible for the observed deviations from the ideal gas law at loftier pressures.
In this section, nosotros explicitly consider 3 kinds of intermolecular interactions.There are two additional types of electrostatic interaction that you are already familiar with: the ion–ion interactions that are responsible for ionic bonding, and the ion–dipole interactions that occur when ionic substances dissolve in a polar substance such as water. The first two are often described collectively every bit van der Waals forces.
Dipole–Dipole Interactions
Polar covalent bonds behave every bit if the bonded atoms have localized fractional charges that are equal but opposite (i.e., the two bonded atoms generate a dipole). If the construction of a molecule is such that the individual bond dipoles do non abolish one another, then the molecule has a cyberspace dipole moment. Molecules with net dipole moments tend to marshal themselves so that the positive end of ane dipole is near the negative end of another and vice versa, every bit shown in Figure \(\PageIndex{1a}\).
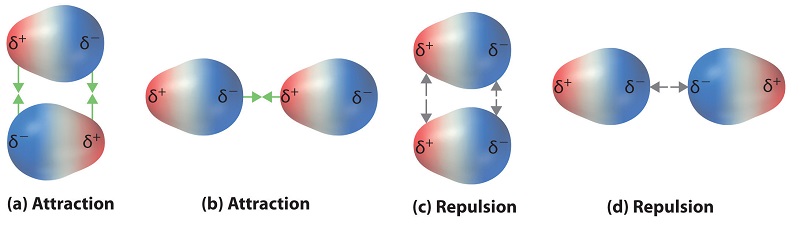
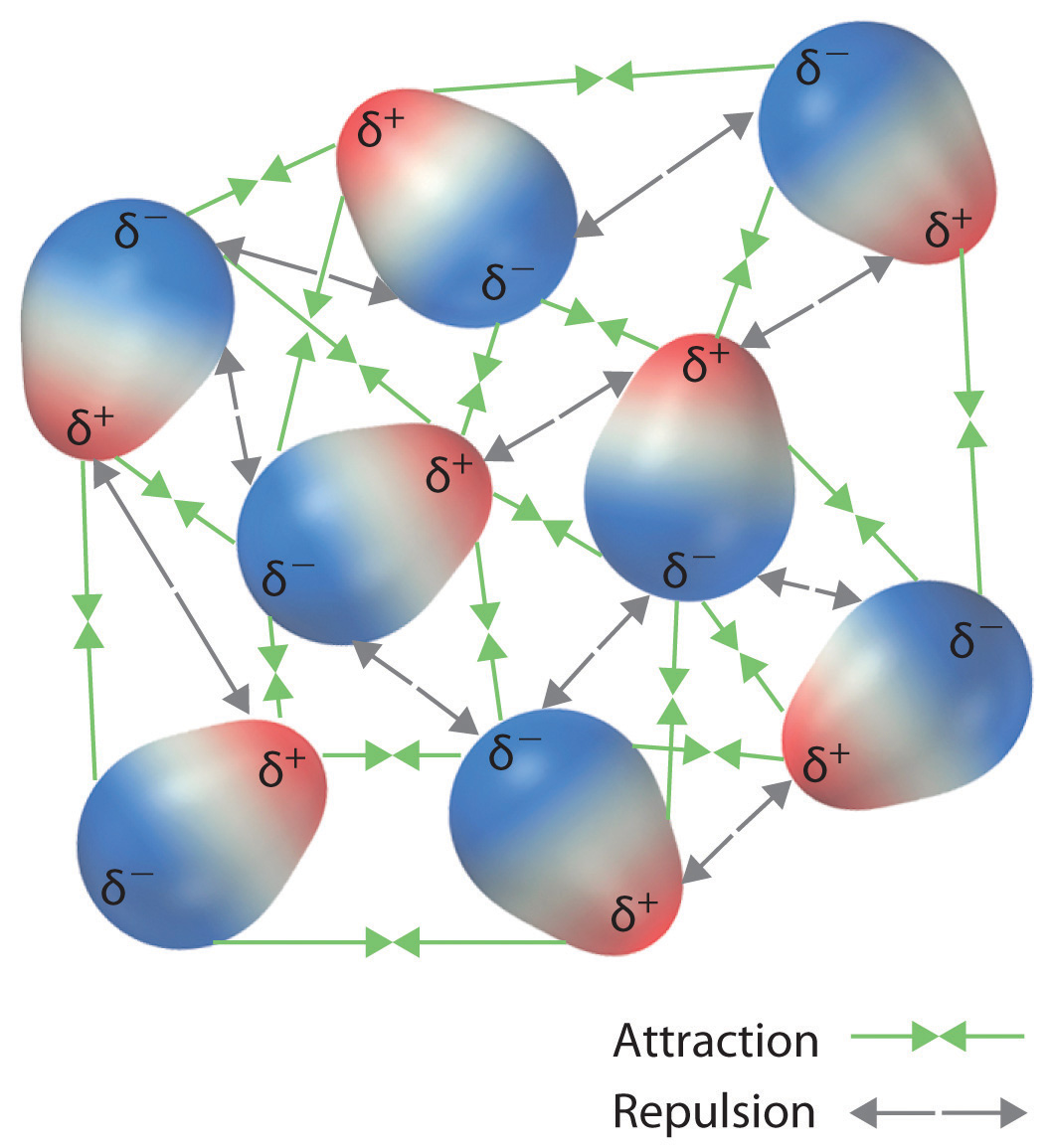
These arrangements are more stable than arrangements in which two positive or two negative ends are adjacent (Figure \(\PageIndex{1c}\)). Hence dipole–dipole interactions, such as those in Figure \(\PageIndex{1b}\), are attractive intermolecular interactions, whereas those in Effigy \(\PageIndex{1d}\) are repulsive intermolecular interactions. Because molecules in a liquid move freely and continuously, molecules e'er feel both attractive and repulsive dipole–dipole interactions simultaneously, as shown in Figure \(\PageIndex{2}\). On average, nonetheless, the bonny interactions boss.
Considering each end of a dipole possesses only a fraction of the charge of an electron, dipole–dipole interactions are substantially weaker than the interactions betwixt ii ions, each of which has a charge of at least ±1, or betwixt a dipole and an ion, in which 1 of the species has at to the lowest degree a total positive or negative charge. In addition, the attractive interaction betwixt dipoles falls off much more than rapidly with increasing distance than exercise the ion–ion interactions. Recall that the attractive energy between two ions is proportional to one/r, where r is the distance between the ions. Doubling the distance (r → 2r) decreases the attractive energy by one-one-half. In dissimilarity, the energy of the interaction of two dipoles is proportional to 1/r 3, and so doubling the distance between the dipoles decreases the force of the interaction past 23, or 8-fold. Thus a substance such equally \(\ce{HCl}\), which is partially held together past dipole–dipole interactions, is a gas at room temperature and 1 atm pressure. Conversely, \(\ce{NaCl}\), which is held together by interionic interactions, is a high-melting-point solid. Within a series of compounds of like molar mass, the strength of the intermolecular interactions increases equally the dipole moment of the molecules increases, equally shown in Tabular array \(\PageIndex{ane}\).
| Compound | Molar Mass (g/mol) | Dipole Moment (D) | Boiling Bespeak (K) |
|---|---|---|---|
| C3Hhalf-dozen (cyclopropane) | 42 | 0 | 240 |
| CH3OCH3 (dimethyl ether) | 46 | 1.xxx | 248 |
| CH3CN (acetonitrile) | 41 | 3.9 | 355 |
The bonny free energy between two ions is proportional to ane/r, whereas the attractive free energy between ii dipoles is proportional to 1/r6.
Video Discussing Dipole Intermolecular Forces. Source: https://youtu.exist/ACq_95SIBck
Example \(\PageIndex{one}\)
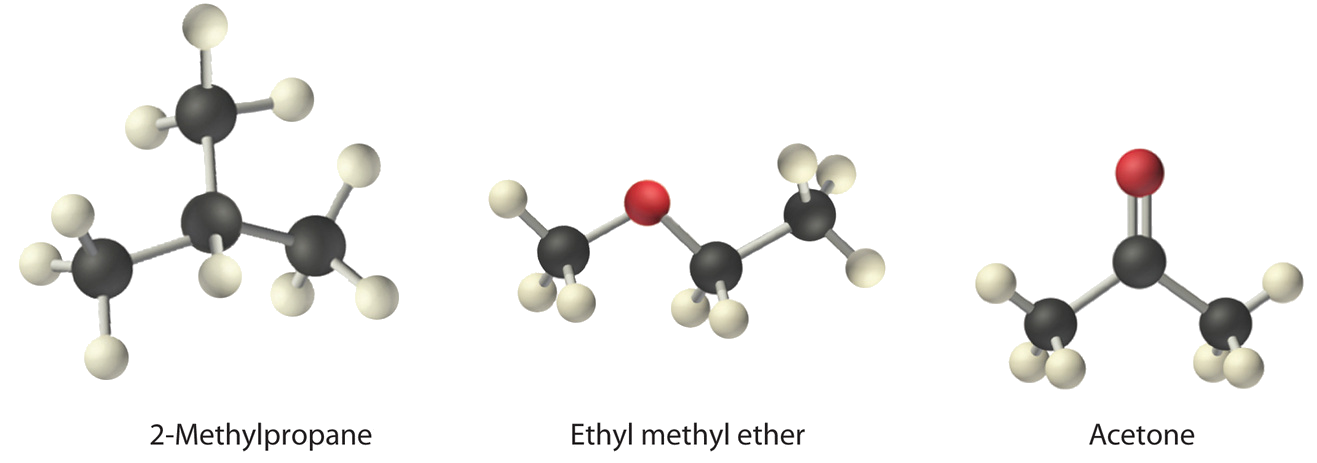
Suit ethyl methyl ether (CH3OCH2CH3), 2-methylpropane [isobutane, (CH3)2CHCH3], and acetone (CHiiiCOCHiii) in guild of increasing boiling points. Their structures are every bit follows:
Given: compounds.
Asked for: social club of increasing humid points.
Strategy:
Compare the tooth masses and the polarities of the compounds. Compounds with higher molar masses and that are polar will have the highest boiling points.
Solution:
The three compounds have essentially the same molar mass (58–sixty 1000/mol), then we must look at differences in polarity to predict the force of the intermolecular dipole–dipole interactions and thus the humid points of the compounds.
The get-go compound, two-methylpropane, contains only C–H bonds, which are not very polar because C and H have similar electronegativities. Information technology should therefore take a very small (but nonzero) dipole moment and a very low boiling point.
Ethyl methyl ether has a structure similar to H2O; it contains ii polar C–O unmarried bonds oriented at about a 109° angle to each other, in addition to relatively nonpolar C–H bonds. As a result, the C–O bail dipoles partially reinforce one another and generate a significant dipole moment that should give a moderately high boiling point.
Acetone contains a polar C=O double bail oriented at about 120° to two methyl groups with nonpolar C–H bonds. The C–O bond dipole therefore corresponds to the molecular dipole, which should outcome in both a rather large dipole moment and a high boiling point.
Thus we predict the post-obit order of boiling points:
2-methylpropane < ethyl methyl ether < acetone
This result is in skilful understanding with the bodily information: two-methylpropane, boiling bespeak = −xi.vii°C, and the dipole moment (μ) = 0.13 D; methyl ethyl ether, boiling point = vii.four°C and μ = ane.17 D; acetone, humid point = 56.i°C and μ = 2.88 D.
Exercise \(\PageIndex{1}\)
Accommodate carbon tetrafluoride (CF4), ethyl methyl sulfide (CH3SC2Hv), dimethyl sulfoxide [(CHthree)2S=O], and ii-methylbutane [isopentane, (CH3)iiCHCH2CH3] in social club of decreasing humid points.
- Answer
-
dimethyl sulfoxide (boiling signal = 189.9°C) > ethyl methyl sulfide (boiling point = 67°C) > 2-methylbutane (boiling point = 27.8°C) > carbon tetrafluoride (boiling indicate = −128°C)
London Dispersion Forces
Thus far, we have considered only interactions betwixt polar molecules. Other factors must exist considered to explain why many nonpolar molecules, such equally bromine, benzene, and hexane, are liquids at room temperature; why others, such as iodine and naphthalene, are solids. Even the noble gases tin can exist liquefied or solidified at low temperatures, loftier pressures, or both (Tabular array \(\PageIndex{2}\)).
What kind of attractive forces can exist between nonpolar molecules or atoms? This question was answered past Fritz London (1900–1954), a High german physicist who later on worked in the Usa. In 1930, London proposed that temporary fluctuations in the electron distributions within atoms and nonpolar molecules could result in the germination of short-lived instantaneous dipole moments, which produce bonny forces called London dispersion forces betwixt otherwise nonpolar substances.
| Substance | Molar Mass (one thousand/mol) | Melting Bespeak (°C) | Boiling Point (°C) |
|---|---|---|---|
| Ar | xl | −189.4 | −185.9 |
| Xe | 131 | −111.viii | −108.one |
| Due north2 | 28 | −210 | −195.8 |
| O2 | 32 | −218.8 | −183.0 |
| Ftwo | 38 | −219.7 | −188.one |
| Itwo | 254 | 113.vii | 184.4 |
| CH4 | 16 | −182.5 | −161.5 |
Consider a pair of adjacent He atoms, for example. On boilerplate, the ii electrons in each He atom are uniformly distributed effectually the nucleus. Because the electrons are in constant motion, however, their distribution in one atom is likely to be asymmetrical at any given instant, resulting in an instantaneous dipole moment. As shown in part (a) in Figure \(\PageIndex{three}\), the instantaneous dipole moment on one atom can interact with the electrons in an next atom, pulling them toward the positive terminate of the instantaneous dipole or repelling them from the negative end. The net consequence is that the start atom causes the temporary formation of a dipole, called an induced dipole, in the second. Interactions between these temporary dipoles cause atoms to be attracted to one another. These bonny interactions are weak and fall off rapidly with increasing distance. London was able to show with quantum mechanics that the bonny free energy betwixt molecules due to temporary dipole–induced dipole interactions falls off as 1/r 6. Doubling the distance therefore decreases the bonny energy by 2half-dozen, or 64-fold.
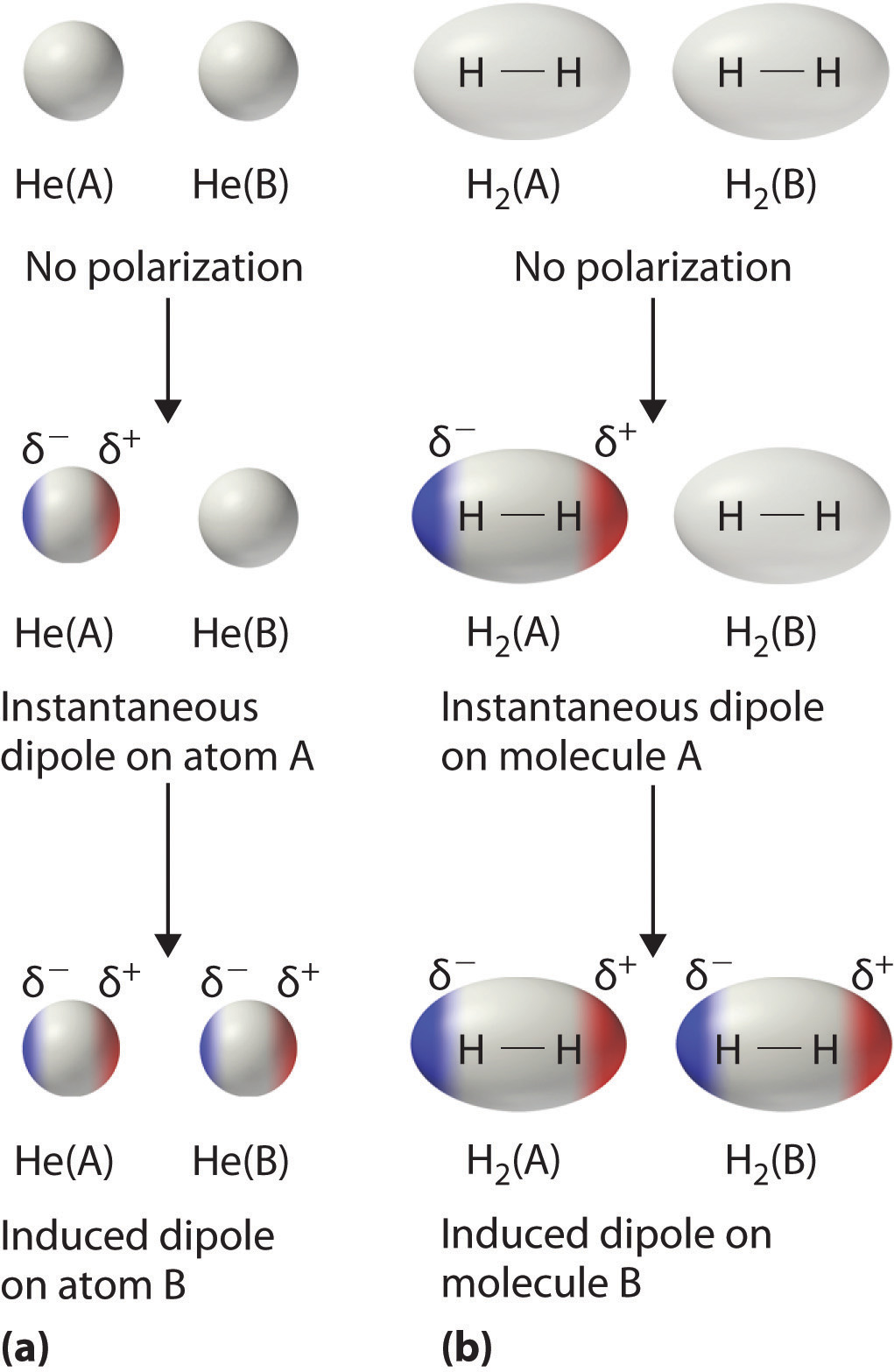
Instantaneous dipole–induced dipole interactions betwixt nonpolar molecules can produce intermolecular attractions just as they produce interatomic attractions in monatomic substances similar Xe. This effect, illustrated for 2 Htwo molecules in role (b) in Figure \(\PageIndex{three}\), tends to become more pronounced as atomic and molecular masses increase (Table \(\PageIndex{2}\)). For example, Xe boils at −108.1°C, whereas He boils at −269°C. The reason for this tendency is that the force of London dispersion forces is related to the ease with which the electron distribution in a given atom tin be perturbed. In small atoms such equally He, the two anesouthward electrons are held close to the nucleus in a very pocket-size book, and electron–electron repulsions are strong enough to prevent significant asymmetry in their distribution. In larger atoms such as Xe, all the same, the outer electrons are much less strongly attracted to the nucleus because of filled intervening shells. As a event, information technology is relatively easy to temporarily deform the electron distribution to generate an instantaneous or induced dipole. The ease of deformation of the electron distribution in an atom or molecule is called its polarizability. Because the electron distribution is more easily perturbed in large, heavy species than in small, light species, we say that heavier substances tend to be much more than polarizable than lighter ones.
For similar substances, London dispersion forces become stronger with increasing molecular size.
The polarizability of a substance also determines how it interacts with ions and species that possess permanent dipoles. Thus, London dispersion forces are responsible for the full general trend toward higher humid points with increased molecular mass and greater surface expanse in a homologous series of compounds, such as the alkanes (part (a) in Figure \(\PageIndex{iv}\)). The strengths of London dispersion forces also depend significantly on molecular shape because shape determines how much of ane molecule tin can interact with its neighboring molecules at whatever given time. For instance, part (b) in Figure \(\PageIndex{4}\) shows two,2-dimethylpropane (neopentane) and n-pentane, both of which have the empirical formula CfiveH12. Neopentane is almost spherical, with a small surface surface area for intermolecular interactions, whereas n-pentane has an extended conformation that enables it to come into close contact with other n-pentane molecules. As a result, the humid point of neopentane (9.5°C) is more than 25°C lower than the boiling point of due north-pentane (36.ane°C).
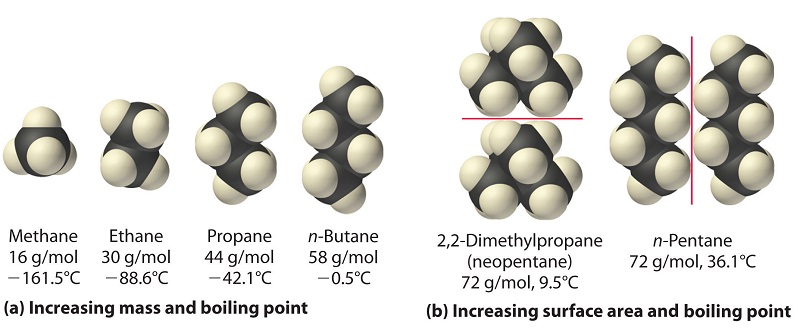
All molecules, whether polar or nonpolar, are attracted to 1 another past London dispersion forces in addition to whatever other attractive forces that may be present. In general, however, dipole–dipole interactions in small polar molecules are significantly stronger than London dispersion forces, and so the onetime predominate.
Video Discussing London/Dispersion Intermolecular Forces. Source: https://youtu.be/RCRTcIEQ-Hk
Example \(\PageIndex{2}\)
Arrange due north-butane, propane, 2-methylpropane [isobutene, (CH3)2CHCH3], and n-pentane in order of increasing boiling points.
Given: compounds
Asked for: order of increasing boiling points
Strategy:
Determine the intermolecular forces in the compounds, and then arrange the compounds co-ordinate to the force of those forces. The substance with the weakest forces will have the lowest humid point.
Solution:
The four compounds are alkanes and nonpolar, so London dispersion forces are the merely important intermolecular forces. These forces are by and large stronger with increasing molecular mass, so propane should have the lowest boiling point and due north-pentane should have the highest, with the two butane isomers falling in between. Of the two butane isomers, two-methylpropane is more compact, and northward-butane has the more than extended shape. Consequently, we expect intermolecular interactions for n-butane to be stronger due to its larger area, resulting in a college boiling point. The overall order is thus every bit follows, with actual boiling points in parentheses: propane (−42.1°C) < 2-methylpropane (−11.7°C) < n-butane (−0.five°C) < northward-pentane (36.ane°C).
Exercise \(\PageIndex{two}\)
Suit GeH4, SiClfour, SiHfour, CH4, and GeCl4 in lodge of decreasing humid points.
- Answer
-
GeCl4 (87°C) > SiCl4 (57.half dozen°C) > GeH4 (−88.5°C) > SiH4 (−111.8°C) > CHiv (−161°C)
Hydrogen Bonds
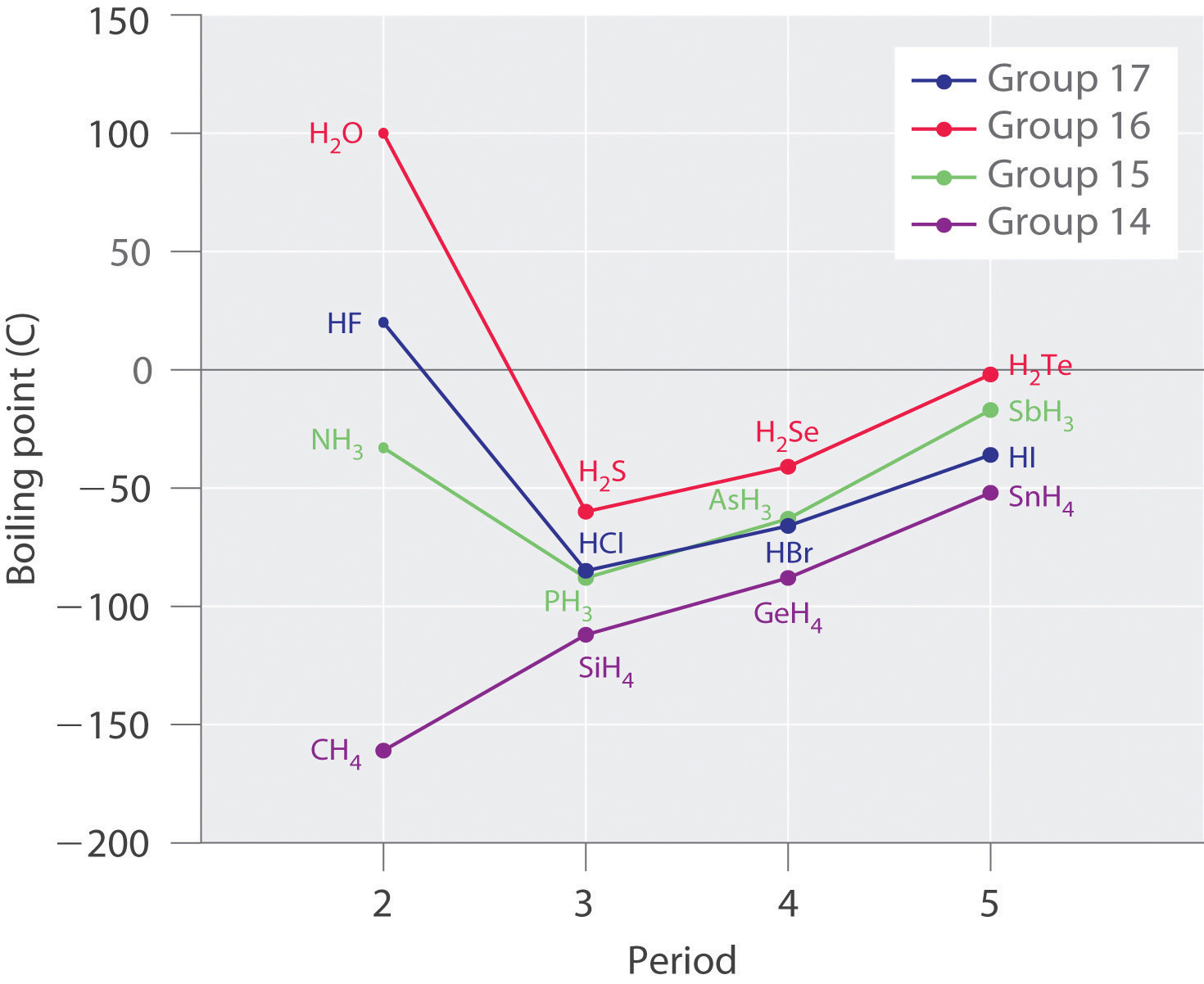
Molecules with hydrogen atoms bonded to electronegative atoms such equally O, N, and F (and to a much lesser extent, Cl and Due south) tend to exhibit unusually stiff intermolecular interactions. These effect in much higher boiling points than are observed for substances in which London dispersion forces dominate, as illustrated for the covalent hydrides of elements of groups 14–17 in Figure \(\PageIndex{5}\). Methyl hydride and its heavier congeners in group 14 form a series whose boiling points increase smoothly with increasing molar mass. This is the expected trend in nonpolar molecules, for which London dispersion forces are the exclusive intermolecular forces. In dissimilarity, the hydrides of the lightest members of groups 15–17 take boiling points that are more than 100°C greater than predicted on the ground of their tooth masses. The upshot is near dramatic for h2o: if nosotros extend the directly line connecting the points for H2Te and HiiSe to the line for period ii, we obtain an estimated boiling point of −130°C for water! Imagine the implications for life on Earth if water boiled at −130°C rather than 100°C.
Why do strong intermolecular forces produce such anomalously high boiling points and other unusual backdrop, such as high enthalpies of vaporization and loftier melting points? The answer lies in the highly polar nature of the bonds betwixt hydrogen and very electronegative elements such as O, Due north, and F. The large difference in electronegativity results in a large partial positive accuse on hydrogen and a correspondingly large partial negative accuse on the O, N, or F atom. Consequently, H–O, H–N, and H–F bonds have very large bond dipoles that can interact strongly with ane another. Because a hydrogen atom is so small, these dipoles can also approach one some other more closely than most other dipoles. The combination of large bond dipoles and short dipole–dipole distances results in very stiff dipole–dipole interactions called hydrogen bonds, equally shown for water ice in Figure \(\PageIndex{6}\). A hydrogen bail is usually indicated past a dotted line between the hydrogen cantlet attached to O, Due north, or F (the hydrogen bond donor) and the atom that has the solitary pair of electrons (the hydrogen bond acceptor). Because each water molecule contains 2 hydrogen atoms and two lonely pairs, a tetrahedral organisation maximizes the number of hydrogen bonds that can be formed. In the construction of ice, each oxygen cantlet is surrounded by a distorted tetrahedron of hydrogen atoms that form bridges to the oxygen atoms of adjacent water molecules. The bridging hydrogen atoms are not equidistant from the two oxygen atoms they connect, nonetheless. Instead, each hydrogen atom is 101 pm from one oxygen and 174 pm from the other. In contrast, each oxygen cantlet is bonded to two H atoms at the shorter altitude and two at the longer altitude, respective to two O–H covalent bonds and two O⋅⋅⋅H hydrogen bonds from side by side water molecules, respectively. The resulting open, cagelike structure of ice ways that the solid is actually slightly less dense than the liquid, which explains why water ice floats on water, rather than sinks.
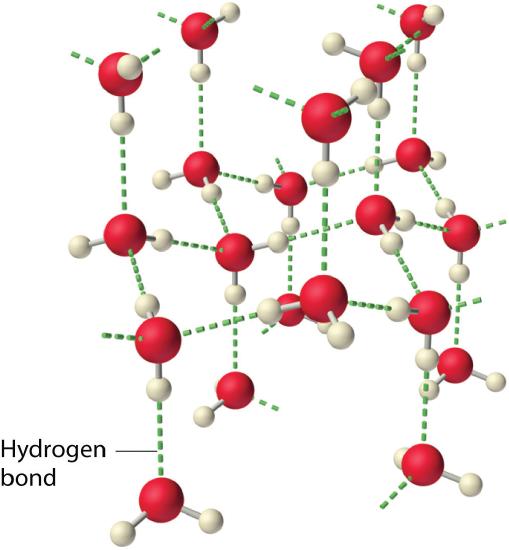
Each water molecule accepts two hydrogen bonds from two other water molecules and donates 2 hydrogen atoms to form hydrogen bonds with 2 more water molecules, producing an open up, cagelike structure. The structure of liquid water is very like, only in the liquid, the hydrogen bonds are continually cleaved and formed considering of rapid molecular movement.
Hydrogen bond germination requires both a hydrogen bond donor and a hydrogen bond acceptor.
Because water ice is less dumbo than liquid water, rivers, lakes, and oceans freeze from the top down. In fact, the ice forms a protective surface layer that insulates the residue of the h2o, assuasive fish and other organisms to survive in the lower levels of a frozen lake or sea. If ice were denser than the liquid, the ice formed at the surface in cold weather would sink as fast as it formed. Bodies of h2o would freeze from the bottom upwards, which would be lethal for nigh aquatic creatures. The expansion of water when freezing besides explains why automobile or boat engines must be protected by "antifreeze" and why unprotected pipes in houses break if they are allowed to freeze.
Video Discussing Hydrogen Bonding Intermolecular Forces. Source: https://youtu.be/92rbjSpHbr0
Example \(\PageIndex{3}\)
Considering CHiiiOH, C2Hhalf dozen, Xe, and (CHthree)threeDue north, which can form hydrogen bonds with themselves? Draw the hydrogen-bonded structures.
Given: compounds
Asked for: germination of hydrogen bonds and construction
Strategy:
- Identify the compounds with a hydrogen atom attached to O, North, or F. These are likely to be able to deed as hydrogen bond donors.
- Of the compounds that tin can act as hydrogen bail donors, place those that also comprise lone pairs of electrons, which permit them to be hydrogen bond acceptors. If a substance is both a hydrogen donor and a hydrogen bail acceptor, draw a structure showing the hydrogen bonding.
Solution:
A. Of the species listed, xenon (Xe), ethane (CiiHvi), and trimethylamine [(CH3)3N] do not contain a hydrogen atom attached to O, Due north, or F; hence they cannot act as hydrogen bond donors.
B. The 1 compound that tin act as a hydrogen bail donor, methanol (CH3OH), contains both a hydrogen atom fastened to O (making it a hydrogen bail donor) and ii lone pairs of electrons on O (making it a hydrogen bond acceptor); methanol tin can thus course hydrogen bonds by acting every bit either a hydrogen bond donor or a hydrogen bond acceptor. The hydrogen-bonded structure of methanol is equally follows:
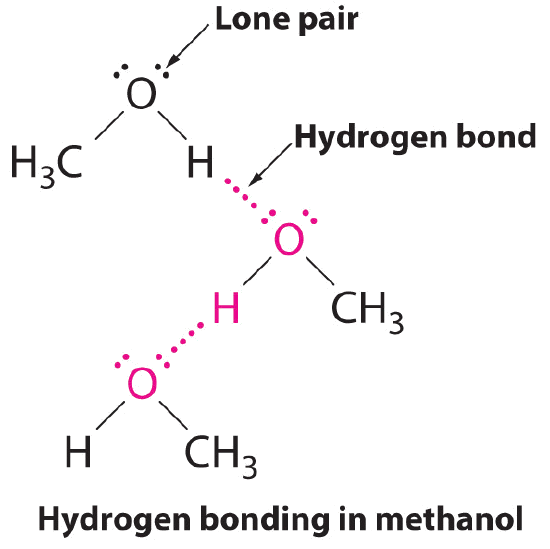
Exercise \(\PageIndex{3}\)
Considering CHiiiCOtwoH, (CH3)iiiN, NHthree, and CH3F, which can form hydrogen bonds with themselves? Draw the hydrogen-bonded structures.
- Answer
-
CH3COtwoH and NHthree;

Although hydrogen bonds are significantly weaker than covalent bonds, with typical dissociation energies of only xv–25 kJ/mol, they have a significant influence on the physical backdrop of a compound. Compounds such every bit HF can form only ii hydrogen bonds at a time every bit can, on boilerplate, pure liquid NH3. Consequently, even though their molecular masses are like to that of water, their humid points are significantly lower than the boiling point of water, which forms four hydrogen bonds at a time.
Example \(\PageIndex{4}\): Buckyballs
Arrange C60 (buckminsterfullerene, which has a muzzle structure), NaCl, He, Ar, and Northward2O in order of increasing boiling points.
Given: compounds.
Asked for: gild of increasing humid points.
Strategy:
Identify the intermolecular forces in each chemical compound then accommodate the compounds co-ordinate to the strength of those forces. The substance with the weakest forces will have the lowest humid signal.
Solution:
Electrostatic interactions are strongest for an ionic compound, and so we expect NaCl to accept the highest boiling point. To predict the relative boiling points of the other compounds, nosotros must consider their polarity (for dipole–dipole interactions), their power to course hydrogen bonds, and their molar mass (for London dispersion forces). Helium is nonpolar and by far the lightest, and so information technology should take the lowest boiling point. Argon and NtwoO have very similar molar masses (40 and 44 g/mol, respectively), only Due north2O is polar while Ar is not. Consequently, N2O should have a higher boiling indicate. A C60 molecule is nonpolar, but its molar mass is 720 grand/mol, much greater than that of Ar or N2O. Because the humid points of nonpolar substances increase rapidly with molecular mass, C60 should boil at a higher temperature than the other nonionic substances. The predicted gild is thus as follows, with actual boiling points in parentheses:
He (−269°C) < Ar (−185.vii°C) < NiiO (−88.5°C) < Csixty (>280°C) < NaCl (1465°C).
Exercise \(\PageIndex{4}\)
Arrange 2,4-dimethylheptane, Ne, CS2, Cltwo, and KBr in order of decreasing boiling points.
- Respond
-
KBr (1435°C) > 2,4-dimethylheptane (132.9°C) > CS2 (46.vi°C) > Cl2 (−34.6°C) > Ne (−246°C)
Example \(\PageIndex{5}\):
Identify the virtually significant intermolecular force in each substance.
- C iii H 8
- CH 3 OH
- H 2 Southward
Solution
a. Although C–H bonds are polar, they are only minimally polar. The most meaning intermolecular force for this substance would be dispersion forces.
b. This molecule has an H atom bonded to an O atom, then it volition experience hydrogen bonding.
c. Although this molecule does not experience hydrogen bonding, the Lewis electron dot diagram and VSEPR betoken that it is bent, and then information technology has a permanent dipole. The most significant force in this substance is dipole-dipole interaction.
Exercise \(\PageIndex{6}\)
Identify the most significant intermolecular force in each substance.
- HF
- HCl
- Answer a
-
hydrogen bonding
- Reply b
-
dipole-dipole interactions
Summary
Intermolecular forces are electrostatic in nature and include van der Waals forces and hydrogen bonds. Molecules in liquids are held to other molecules by intermolecular interactions, which are weaker than the intramolecular interactions that concur the atoms together inside molecules and polyatomic ions. Transitions between the solid and liquid, or the liquid and gas phases, are due to changes in intermolecular interactions, but do non impact intramolecular interactions. The three major types of intermolecular interactions are dipole–dipole interactions, London dispersion forces (these two are often referred to collectively as van der Waals forces), and hydrogen bonds. Dipole–dipole interactions arise from the electrostatic interactions of the positive and negative ends of molecules with permanent dipole moments; their strength is proportional to the magnitude of the dipole moment and to 1/riii , where r is the distance between dipoles. London dispersion forces are due to the formation of instantaneous dipole moments in polar or nonpolar molecules equally a result of short-lived fluctuations of electron charge distribution, which in turn cause the temporary germination of an induced dipole in next molecules; their free energy falls off every bit 1/r vi. Larger atoms tend to exist more polarizable than smaller ones, because their outer electrons are less tightly bound and are therefore more easily perturbed. Hydrogen bonds are particularly stiff dipole–dipole interactions between molecules that accept hydrogen bonded to a highly electronegative atom, such every bit O, N, or F. The resulting partially positively charged H atom on i molecule (the hydrogen bond donor) can interact strongly with a lone pair of electrons of a partially negatively charged O, N, or F cantlet on side by side molecules (the hydrogen bond acceptor). Because of strong O⋅⋅⋅H hydrogen bonding between water molecules, water has an unusually loftier humid point, and ice has an open up, cagelike structure that is less dumbo than liquid water.
holbrookpeatchath.blogspot.com
Source: https://chem.libretexts.org/Bookshelves/General_Chemistry/Map%3A_Chemistry_-_The_Central_Science_(Brown_et_al.)/11%3A_Liquids_and_Intermolecular_Forces/11.2%3A_Intermolecular_Forces
0 Response to "what intermolecular forces must be overcome to melt solid i2?"
Post a Comment